Welcome to the exciting collaboration between Hydreight and Semaglutide, where innovative marketing strategies meet groundbreaking pharmaceutical developments. Come join us in reshaping the way we approach metabolic management and weight loss support.
Together, let's pave the way for a healthier future.
Are you eager to learn more about capitalizing on this groundbreaking opportunity to generate income?
Hydreight: How to Market Your Mobile Services
Introducing Semaglutide
One notable addition to our platform is the introduction of Semaglutide, a groundbreaking release poised to revolutionize the industry. This presents an exciting opportunity for businesses to incorporate this innovative product into their offerings and sometimes businesses need a little extra push to reach their full potential.
Whether it's fine-tuning your sales strategy or providing personalized support, we're dedicated to helping you unlock new opportunities and achieve your goals.

.png?width=2000&height=1545&name=Marketing%20Content%20(1).png)
Educational Content
- Micromedex Drug Detail re Semaglutide – Drugdex Evaluations dated 2/14/23.
- NOVOMEDLINK. Obesity. (2022).
- Ojeniran, M., Dube, B., Paige, A., Ton, J., & Lindblad, A. J. (2021). Semaglutide for weight loss. Canadian family physician Medecin de famille canadien, 67(11), 842.
- Newsome, Philip N., et als., A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. New England Journal of Medicine, 384:1113-1124
- American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity (2022).
- PubMed
- According to Goldman Sachs, it is projected that by 2030, the global market for anti-obesity medications (AOMs) could grow by more than 16 times to $100 billion. Based on current trends, over half of the global population will be overweight or obese by 2035, compared to 38% in 2020, according to a World Obesity Atlas 2023 forecast.
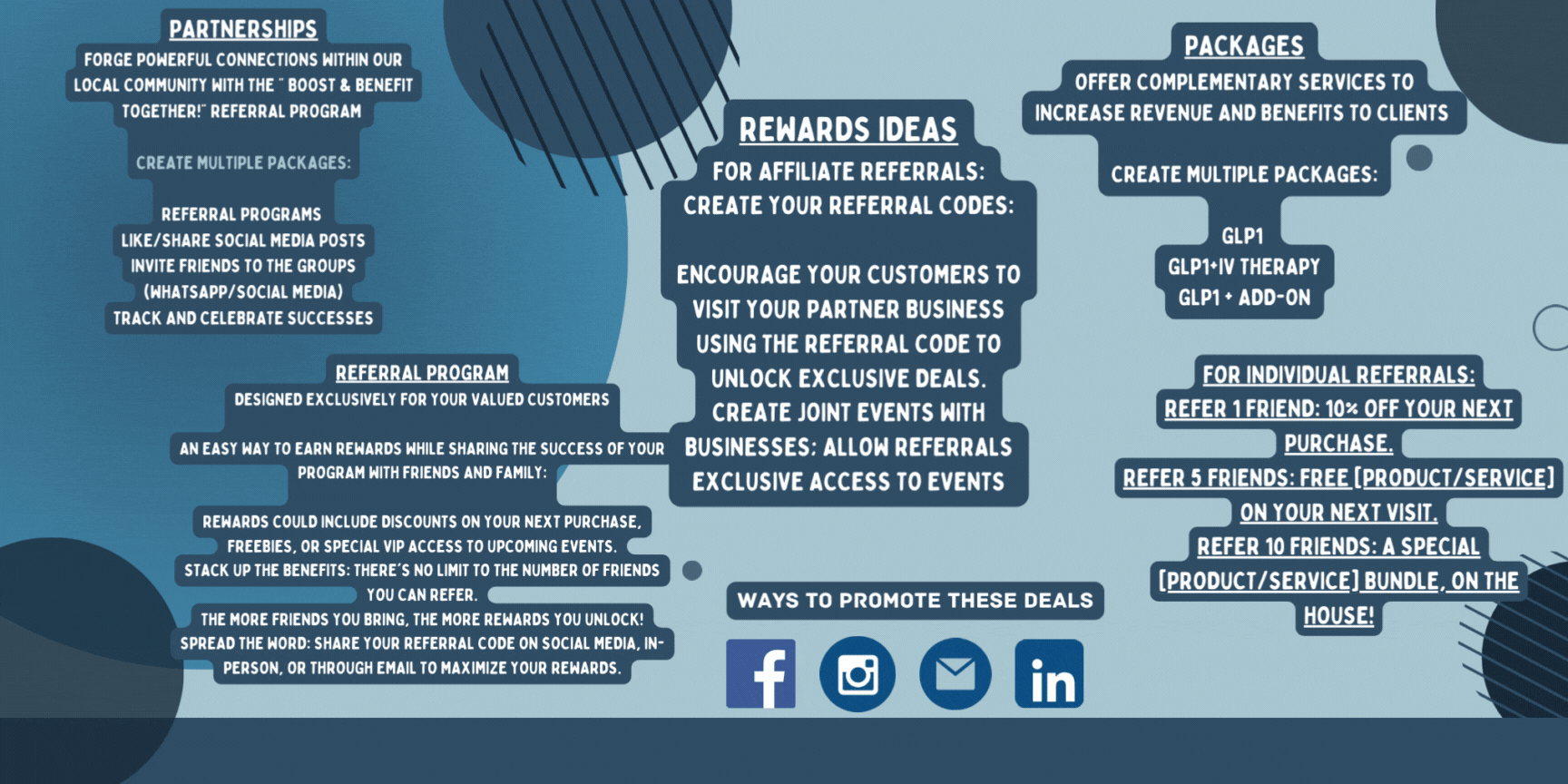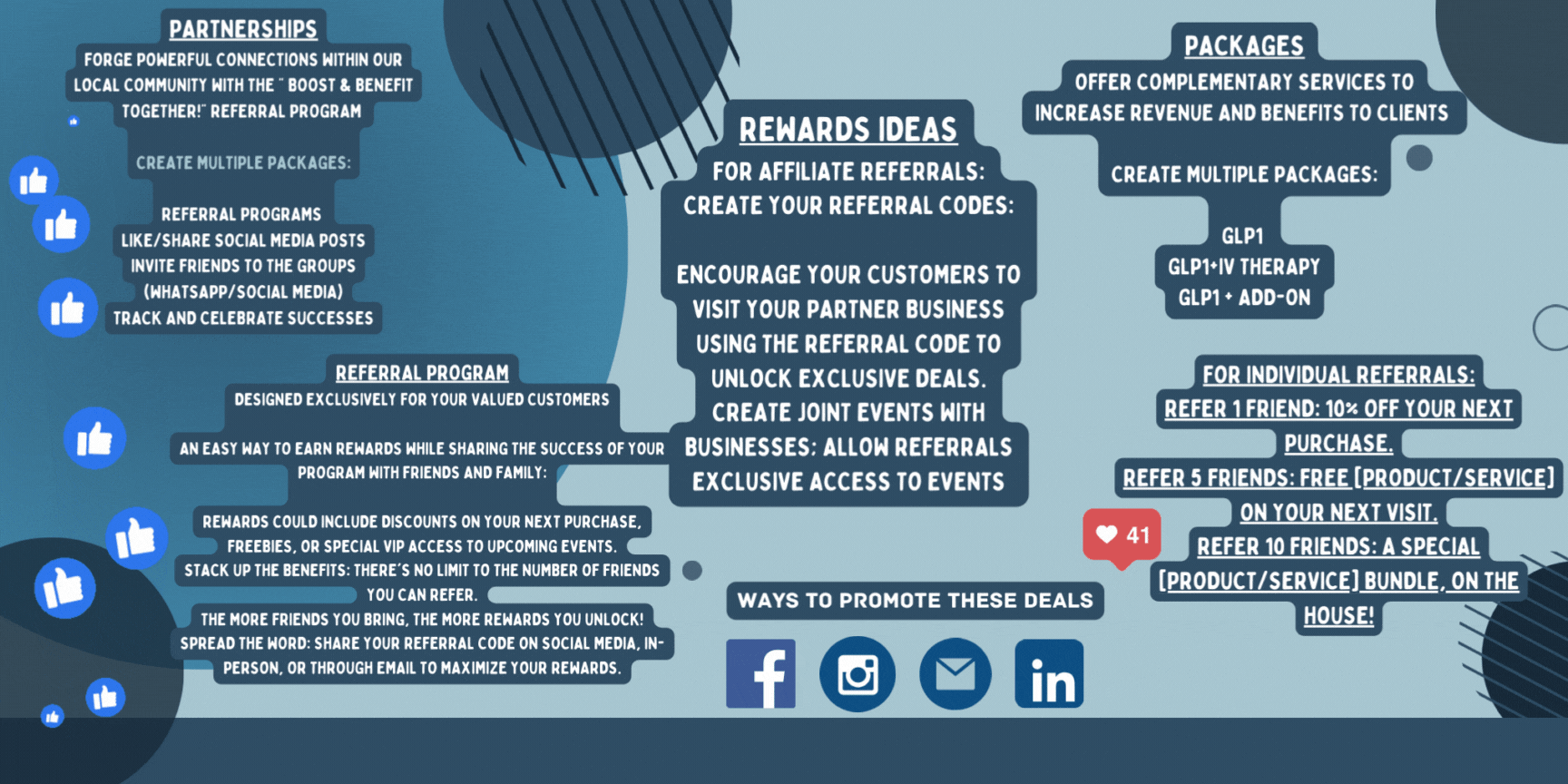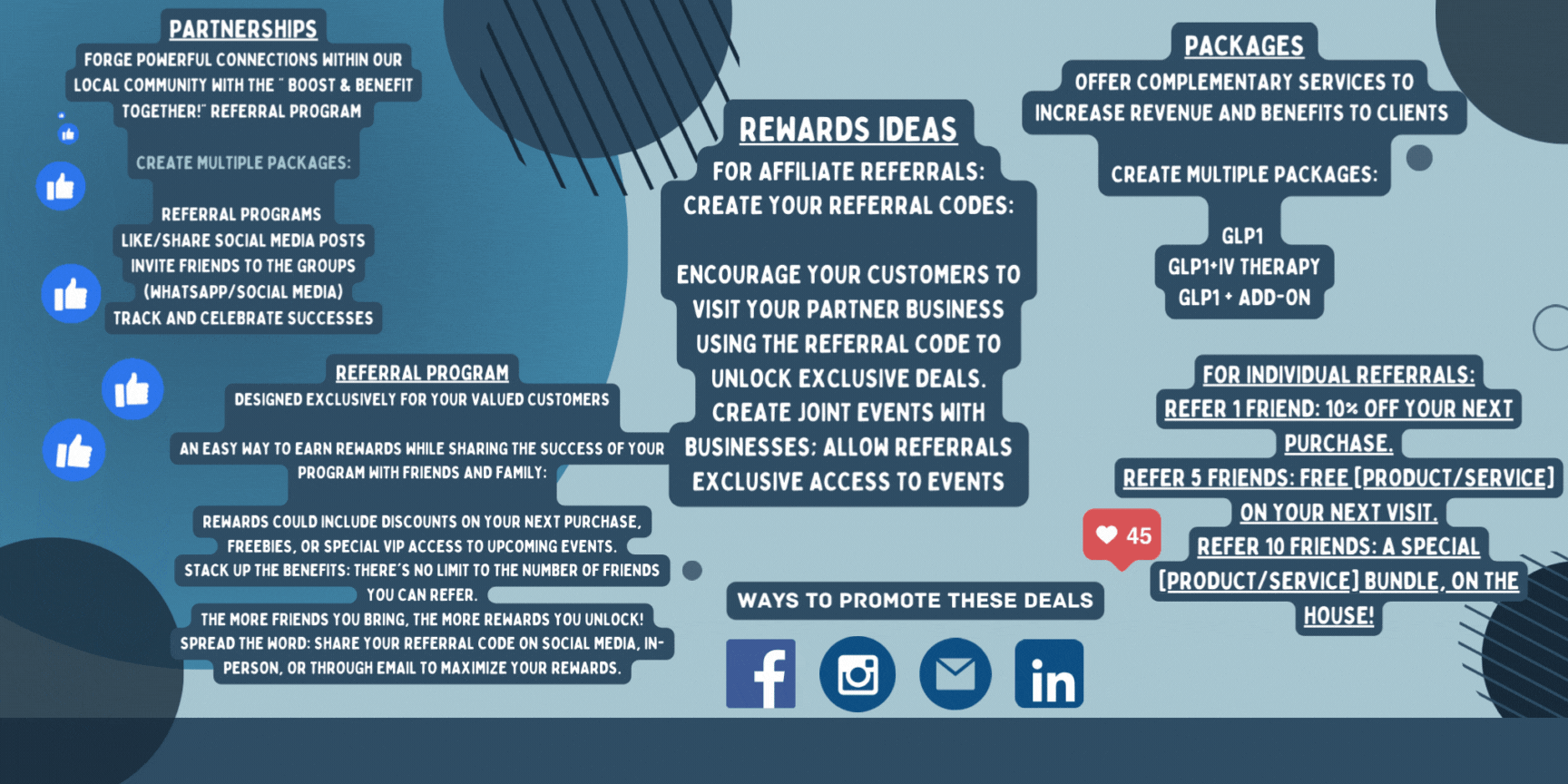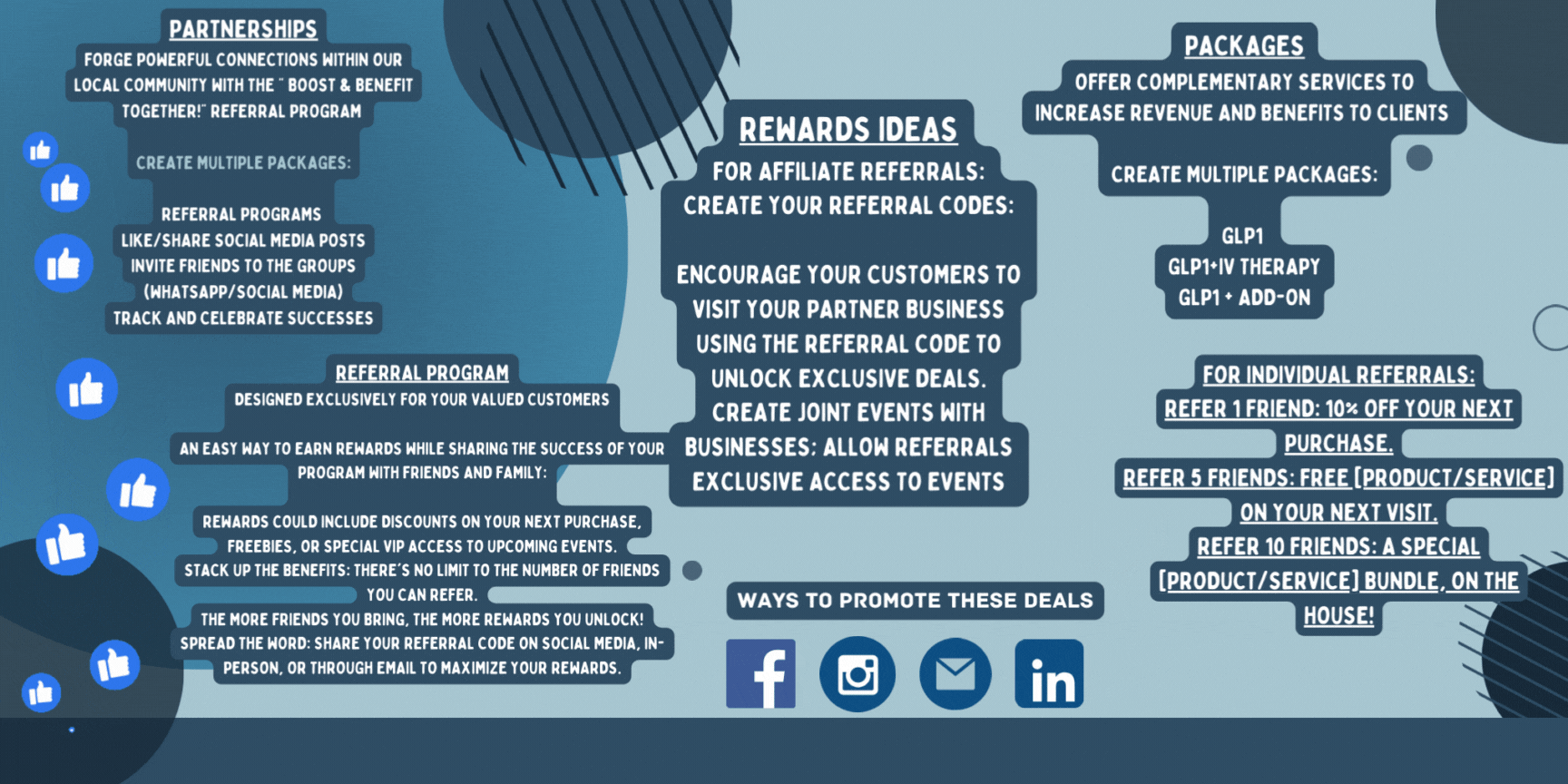
Marketing Partnerships

.png)

